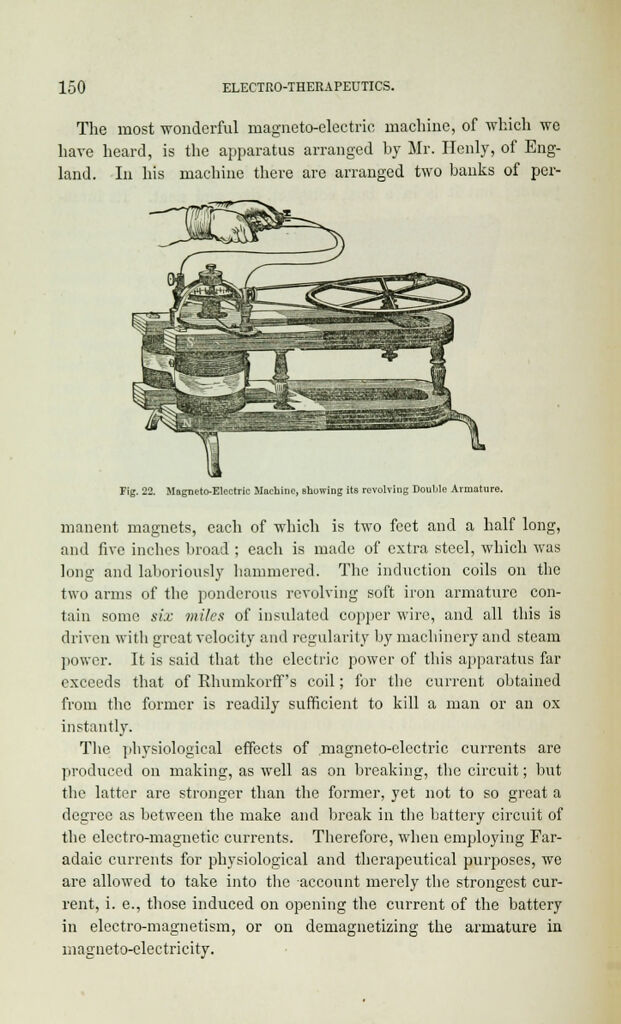
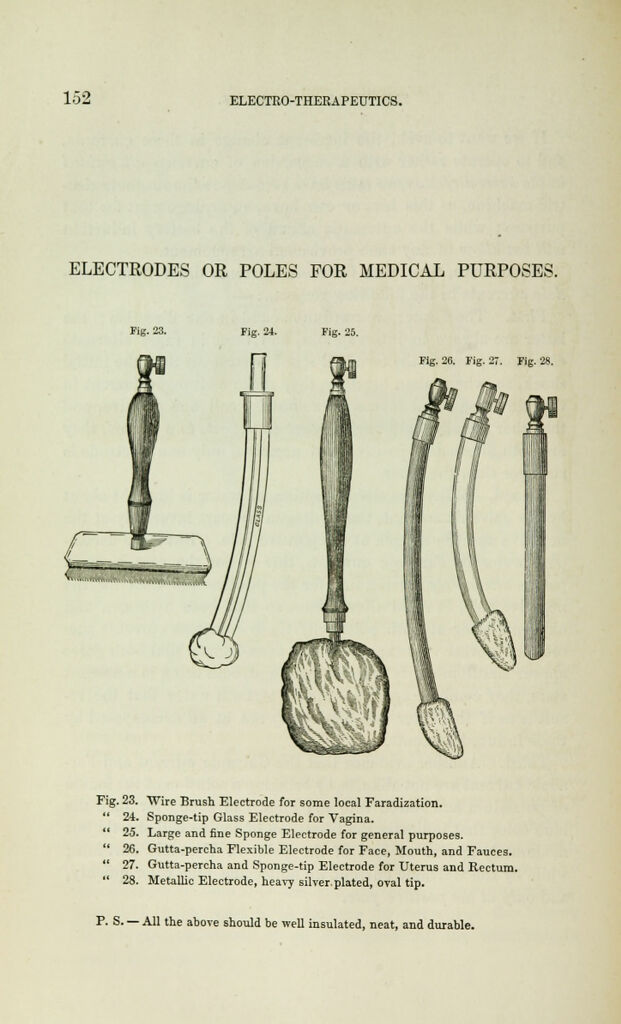
Electro-physiology and electro-therapeutics : showing the best methods for the medical uses of electricity / By Alfred C. Garratt.
166/740
- Publication/creation
- Boston : Ticknor and Fields, 1861.
- Physical description
- 3 unnumbered pages, 716 pages, 2 leaves of plates : illustrations ; 26 cm
- Contributors
-
Garratt, Alfred C. (Alfred Charles), 1813?-1891
Harvey Cushing/John Hay Whitney Medical Library
- Notes
-
3d ed. has title: Medical electricity
Includes index and testimonials
- Type/technique
- Electronic books
- Subjects
-
Electrophysiology
Electrotherapeutics
- Attribution and usage
-
This material has been provided by the Harvey Cushing/John Hay Whitney Medical Library at Yale University, through the Medical Heritage Library. The original may be consulted at the Harvey Cushing/John Hay Whitney Medical Library at Yale University.
This work has been identified as being free of known restrictions under copyright law, including all related and neighbouring rights and is being made available under the Creative Commons, Public Domain Mark.
You can copy, modify, distribute and perform the work, even for commercial purposes, without asking permission.
The image contains the following text:
If we want to avoid the incessant change in these currents,
and to operate rather with a succession of currents all guided
in the same direction, we must have recourse to the magneto-elec-
tric machine, as this has, or can have, an arrangement for that
purpose ; while the automatic action of the battery induction
ivill not allow of any such provisional arrangement.
We observe, then, that Galvanic currents differ from Fara-
daic currents in the following respects : —
First. The former are continuous and in one direction ; the
latter are always in interruptions, and these in rapid alternate
directions ; but as the terminal shock is stronger than the initial
shock, so, when taken together, they make a stronger current in
that direction; and hence we nominally call one positive, and
the other negative, although in fact and effect, to a degree, they
are both alternately positive and negative, only one electrode is
stronger than the other.
Second. When the decomposition of water is brought about
by the galvanic current, the hydrogen appears invariably at the
negative and the oxygen at the positive pole. But if we decom-
pose water by Faradaic currents, this is not the case, as each
pole is alternate/// serving first for the positive and then for the
negative pole, in rapid alternations, so that both hydrogen and
oxygen appear at both poles. If these induction currents suc-
ceed each other very rapidly, it may even happen that both gases
appear simultaneously at either pole, and, both being in a nascent
state, they combine again so rapidly to form water that the re-
sult is as if the water was apparently not at all decomposed by
these induced currents.
Third. Another evidence that the Galvanic current and Far-
adaic current are not alike, is, by bringing a solution of the iodide
of potassium and starch into the circuit of each ; for then the
blue color that indicates the liberation of iodine will shortly but
moderately appear at both of the poles of Faradaic currents;
while by the Galvanic current we notice the blue color quickly,
and only at the positive pole.