Electro-physiology and electro-therapeutics : showing the best methods for the medical uses of electricity / By Alfred C. Garratt.
120/740
- Publication/creation
- Boston : Ticknor and Fields, 1861.
- Physical description
- 3 unnumbered pages, 716 pages, 2 leaves of plates : illustrations ; 26 cm
- Contributors
-
Garratt, Alfred C. (Alfred Charles), 1813?-1891
Harvey Cushing/John Hay Whitney Medical Library
- Notes
-
3d ed. has title: Medical electricity
Includes index and testimonials
- Type/technique
- Electronic books
- Subjects
-
Electrophysiology
Electrotherapeutics
- Attribution and usage
-
This material has been provided by the Harvey Cushing/John Hay Whitney Medical Library at Yale University, through the Medical Heritage Library. The original may be consulted at the Harvey Cushing/John Hay Whitney Medical Library at Yale University.
This work has been identified as being free of known restrictions under copyright law, including all related and neighbouring rights and is being made available under the Creative Commons, Public Domain Mark.
You can copy, modify, distribute and perform the work, even for commercial purposes, without asking permission.
The image contains the following text:
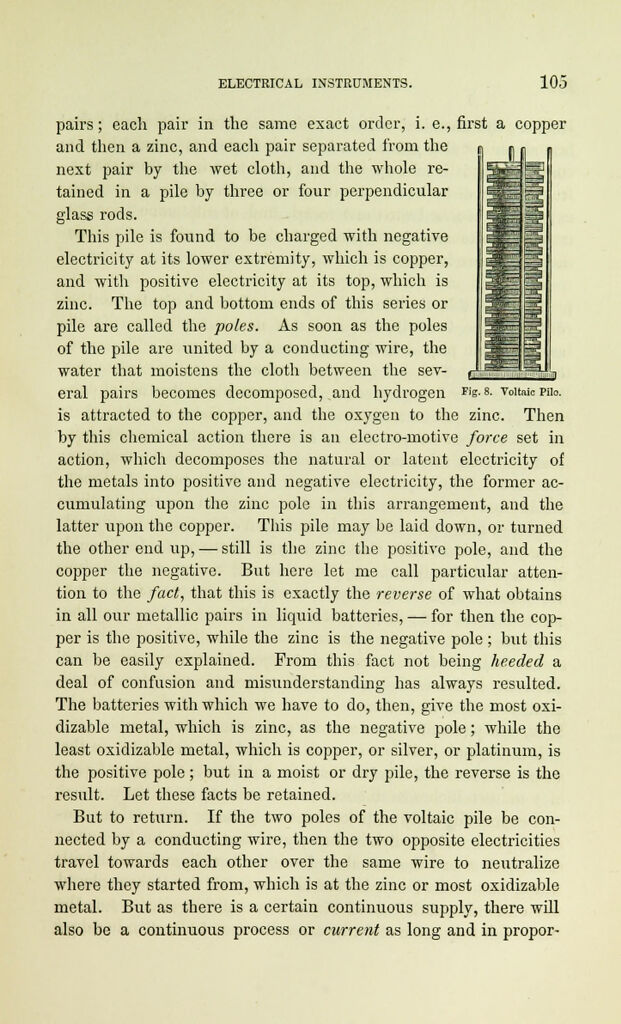
pairs; each pair in the same exact order, i. e., first a copper
and then a zinc, and each pair separated from the
next pair by the wet cloth, and the whole re-
tained in a pile by three or four perpendicular
glass rods.
This pile is found to be charged with negative
electricity at its lower extremity, which is copper,
and with positive electricity at its top, which is
zinc. The top and bottom ends of this series or
pile are called the poles. As soon as the poles
of the pile are united by a conducting wire, the
water that moistens the cloth between the sev-
eral pairs becomes decomposed, and hydrogen ris-8- voitaicPiio.
is attracted to the copper, and the oxygen to the zinc. Then
by this chemical action there is an electro-motive force set in
action, which decomposes the natural or latent electricity of
the metals into positive and negative electricity, the former ac-
cumulating upon the zinc pole in this arrangement, and the
latter upon the copper. This pile may be laid clown, or turned
the other end up, — still is the zinc the positive pole, and the
copper the negative. But here let me call particular atten-
tion to the fact, that this is exactly the reverse of what obtains
in all our metallic pairs in liquid batteries, — for then the cop-
per is the positive, while the zinc is the negative pole ; but this
can be easily explained. From this fact not being heeded a
deal of confusion and misunderstanding has always resulted.
The batteries with which we have to do, then, give the most oxi-
dizable metal, which is zinc, as the negative pole; while the
least oxidizable metal, which is copper, or silver, or platinum, is
the positive pole; but in a moist or dry pile, the reverse is the
result. Let these facts be retained.
But to return. If the two poles of the voltaic pile be con-
nected by a conducting wire, then the two opposite electricities
travel towards each other over the same wire to neutralize
where they started from, which is at the zinc or most oxidizable
metal. But as there is a certain continuous supply, there will
also be a continuous process or current as long and in propor-